
Those with the disorder had to rely on unapproved creams, cosmetic solutions and injections to manage their condition, Jonathan Wosen and Akila Muthukumar report for STAT. Until now, no approved treatment existed to make hair grow back in patients with alopecia areata. Roughly 40 percent of those individuals have a severe form of the autoimmune disorder, meaning that they are missing at least half of the hair on their scalp, STAT reports. About 700,000 individuals in the United States are living with alopecia areata. Hair loss is usually found on the head and face but can occur in small, round, coin-shaped patches anywhere on the body, according to the National Institutes of Health. Oluminant was studied in two trials for the treatment of alopecia areata, and the results were published last month in the New England Journal of Medicine.Īlopecia areata is a disease that occurs when the immune system attacks hair follicles, causing hair loss.

The drug was originally developed by the pharmaceutical company Eli Lilly and has already been on the market for about four years for treating rheumatoid arthritis and other autoimmune diseases. The medicine is the first FDA approval of a systemic or full-body drug for the condition, per a statement.
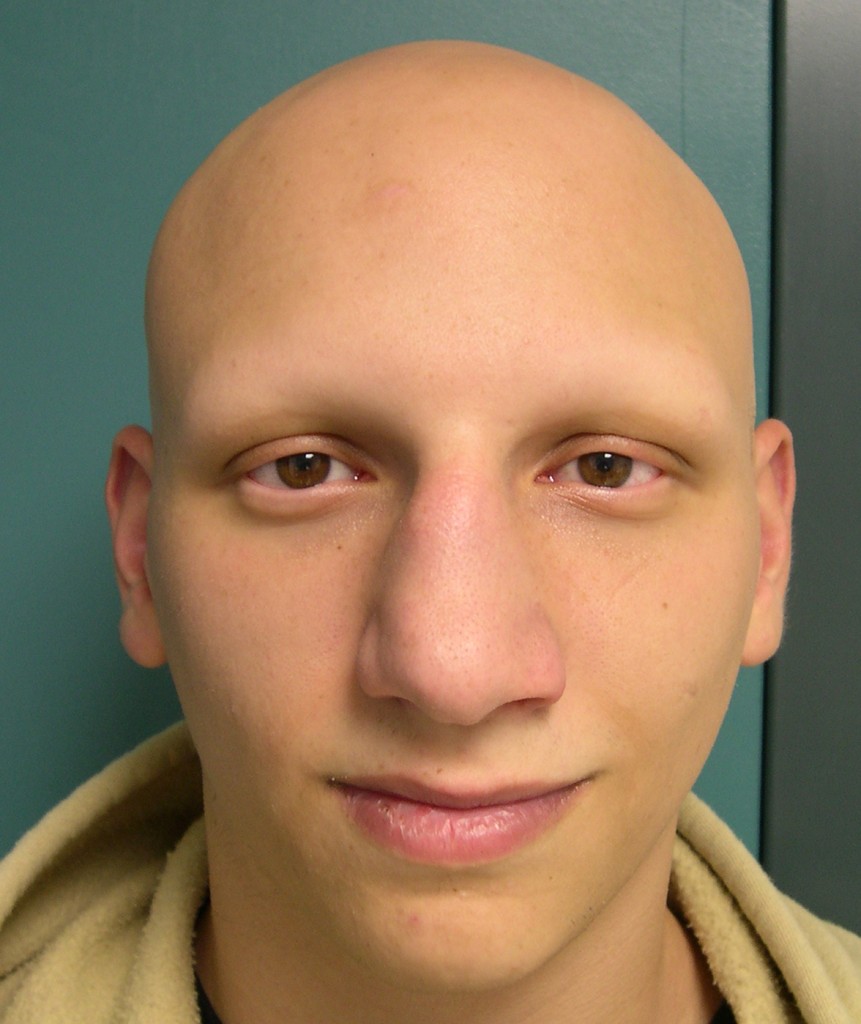
Food and Drug Administration (FDA) has approved the drug Olumiant (baricitinib) for adult patients with severe alopecia areata, an immune disorder that often results in hair loss.


 0 kommentar(er)
0 kommentar(er)
